
Surgical Gowns: A Complete Guide to Selection and Proper Use
In the healthcare sector, surgical gowns represent one of the most important protection barriers for the use of healthcare providers and patients in a surgery room that protects against contamination, infection, and exposure to bodily fluids. Given this, it is important to understand how surgical gowns are designed, what various protection levels they provide, and the factors that should be considered in the selection of the right gown.
Amaryllis Healthcare launched an exclusive range of SecureCare Gowns to protect the healthcare team from the spread of infection during high-risk fluid-intensive procedures. ARAS [Alcohol Repellant and Anti-static] SMS fabric provides best protection as it is treated with fluorochemicals and antistatic agents to enhance resistance to penetration by alcohol-based solutions and to reduce or eliminate the buildup of static electricity during surgery.
What is a Surgical Gown?
A surgical gown is a type of hospital gown designed to be worn by medical professionals and other staff involved during surgeries or procedures to prevent contamination and provide protection against infection. These gowns are regulated by agencies like
- U.S. Food and Drug Administration (FDA): Regulates surgical gowns in the United States to ensure they meet strict safety and performance criteria
- Association for the Advancement of Medical Instrumentation (AAMI):Establishes standards for the protective performance of surgical gowns, including levels of fluid and microbial barrier protection (AAMI PB70 standards)
- Centers for Disease Control and Prevention (CDC): Provides guidelines for the use of personal protective equipment (PPE), including surgical gowns, to prevent the transmission of infectious diseases
- European Union (CE Certification): Surgical gowns sold in Europe must meet the requirements of the European Medical Devices Regulation (MDR) and the EN 13795 standard, which outlines testing and performance criteria
- International Organization for Standardization (ISO): Sets global standards like ISO 13485 for the quality management systems used in the manufacturing of medical devices, including surgical gowns
1. What are the various types of Surgical Gowns?
Surgical Gowns
Surgical gowns can be categorized into two major types:
Sterile Surgical Gowns
- Primary Use: These gowns are essential in surgical environments where a high level of protection is required. They are used by surgeons, nurses, and other operating room personnel.
- Protection Level: Sterile gowns provide a barrier against microbial penetration, protecting both the patient and medical staff from cross-contamination during invasive procedures.
- Sterilization Process: They undergo sterilization (commonly via Ethylene Oxide or radiation) to ensure they are completely free from bacteria, viruses, and other pathogens before use.
- Typical Applications: High-risk procedures, surgeries, or any clinical situation that requires a sterile environment to minimize infection risks.
- Packaging: Individually packaged in sterile wraps to maintain sterility until the gown is ready for use in a controlled setting.
Non-Sterile Surgical Gowns
- Primary Use: These gowns are designed for lower-risk environments where sterility is not critical. They are commonly worn in routine medical procedures, examinations, or for general hospital use.
- Protection Level: While they still offer a barrier against fluids and general contamination, they are not intended to protect against high microbial exposure as sterile gowns do.
- Sterilization: Non-sterile gowns are not treated to eliminate all pathogens but are still manufactured under strict hygiene standards.
- Typical Applications: Non-surgical procedures, routine patient care, or outpatient procedures where the risk of infection is low.
- Packaging: Generally packed in bulk and not individually sealed in sterile packaging.
2. How are Surgical Gowns regulated?
- Primary Use: These gowns are designed for lower-risk environments where sterility is not critical. They are commonly worn in routine medical procedures, examinations, or for general hospital use.
- Protection Level: While they still offer a barrier against fluids and general contamination, they are not intended to protect against high microbial exposure as sterile gowns do.
- Sterilization: Non-sterile gowns are not treated to eliminate all pathogens but are still manufactured under strict hygiene standards.
- Typical Applications: Non-surgical procedures, routine patient care, or outpatient procedures where the risk of infection is low.
- Packaging: Generally packed in bulk and not individually sealed in sterile packaging.
2. How are Surgical Gowns regulated?
Surgical gowns are considered Class II medical devices by the U.S. Food and Drug Administration (FDA), meaning they must meet specific safety and performance standards. These gowns need to provide adequate protection against fluids and infection risks. Here are the key organizations and standards that regulate surgical gowns:
- FDA (U.S. Food and Drug Administration): In the U.S., the FDA ensures surgical gowns are safe and effective by regulating them as Class II medical devices.
- AAMI (Association for the Advancement of Medical Instrumentation): AAMI sets guidelines for the fluid resistance and barrier protection of gowns through its AAMI PB70 standards, which define four levels of protection based on the type of exposure.
- CE Marking (European Union): Gowns sold in Europe must meet the EN 13795 standard under the EU’s Medical Devices Regulation (MDR). This standard focuses on microbial cleanliness and barrier properties.
- ISO (International Organization for Standardization): The ISO 13485 standard governs the quality management systems of manufacturers, ensuring that surgical gowns are consistently produced to meet safety and performance requirements.
- CDC (Centers for Disease Control and Prevention): The CDC provides guidance on the use of personal protective equipment (PPE), including surgical gowns, for preventing infection transmission in healthcare settings.
In simpler terms, surgical gowns must meet strict standards to ensure they provide the right level of protection in healthcare environments. These standards are set by global organizations to safeguard both medical staff and patients.
Access FDA guidelines on surgical gown standards here.
ADVANTAGES OF DISPOSABLE SURGICAL GOWNS
Several studies suggest that the disposable gowns can reduce the risk of surgical site infections (SSIs) by almost 40%. Their superior barrier properties, particularly resistance to fluids and bacteria, make them highly effective in maintaining a sterile environment during procedures. Unlike reusable gowns, which need proper laundering and sterilization, disposables minimize the chance of errors and problems in these processes.
Additionally, one study reported a 26% reduction in wound infections when disposable drapes and gowns were used compared to reusable products. By eliminating the need for reprocessing, disposable gowns help ensure consistent infection control, particularly in high risk surgical environments. The material is specifically designed to meet stringent infection control standards, making them a trusted option in modern healthcare facilities.
This reliability, along with the convenience of single use products, has driven their popularity in the operating rooms, especially in procedures with higher fluid exposure risks.
3. What should you consider when selecting a Surgical Gown?
Many factors should be taken into consideration while selecting the right surgical gown for your needs:
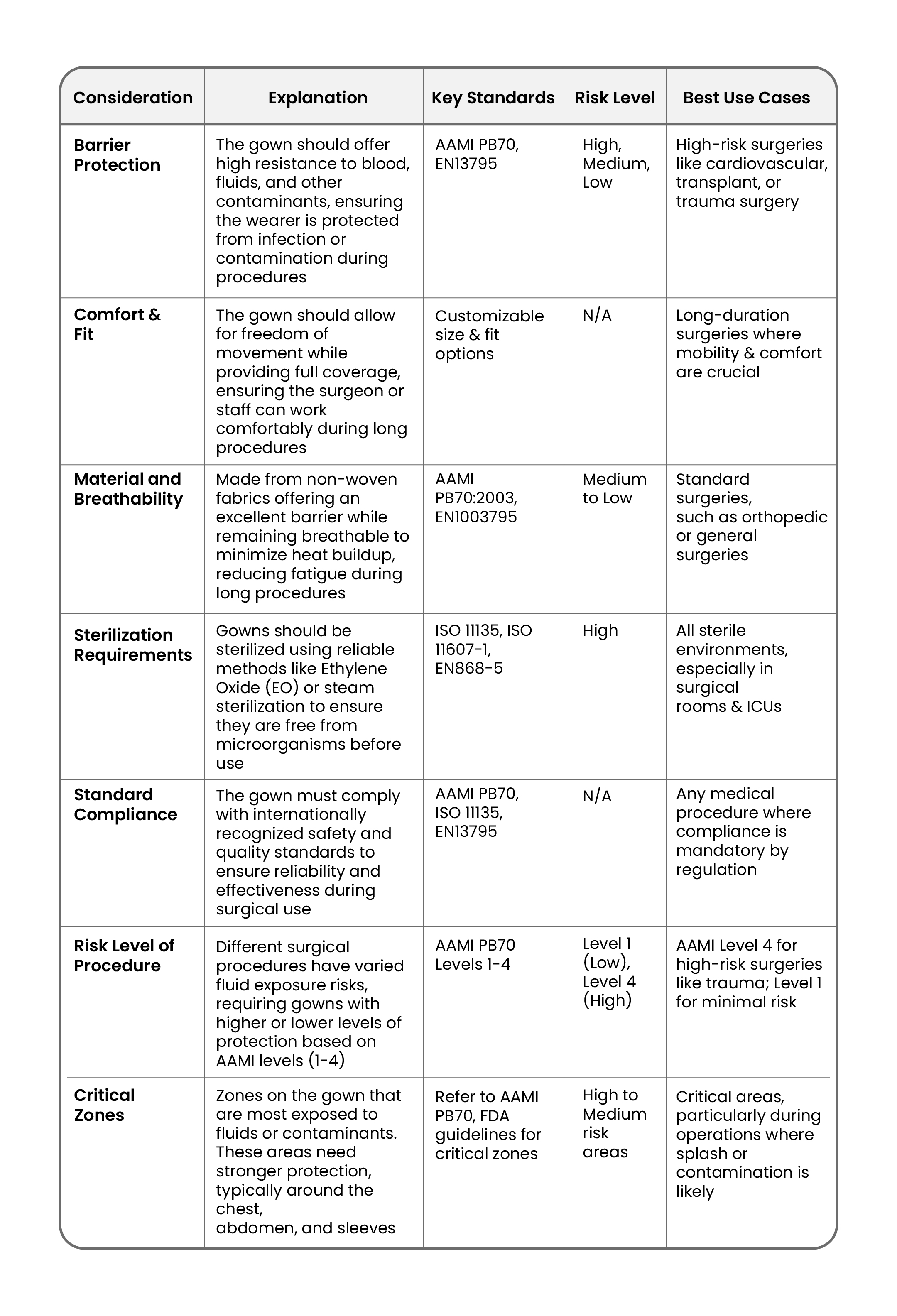
Understanding Critical Zones of Surgical Gowns
While selecting a surgical gown, it is important to be aware of the critical zones—the areas likely to come into contact with fluids and contaminants. According to the FDA and the AAMI guidelines, these critical zones include:
- Front of the gown, from chest to knees
- Sleeves from cuff to above the elbow
View detailed information on critical zones in this document.
AAMI Levels of Protection
Surgical gowns are classified into four levels of protection as defined by the Association for the Advancement of Medical Instrumentation (AAMI). These levels are based on their resistance to fluid penetration and are crucial for determining the right gown for different types of surgeries.
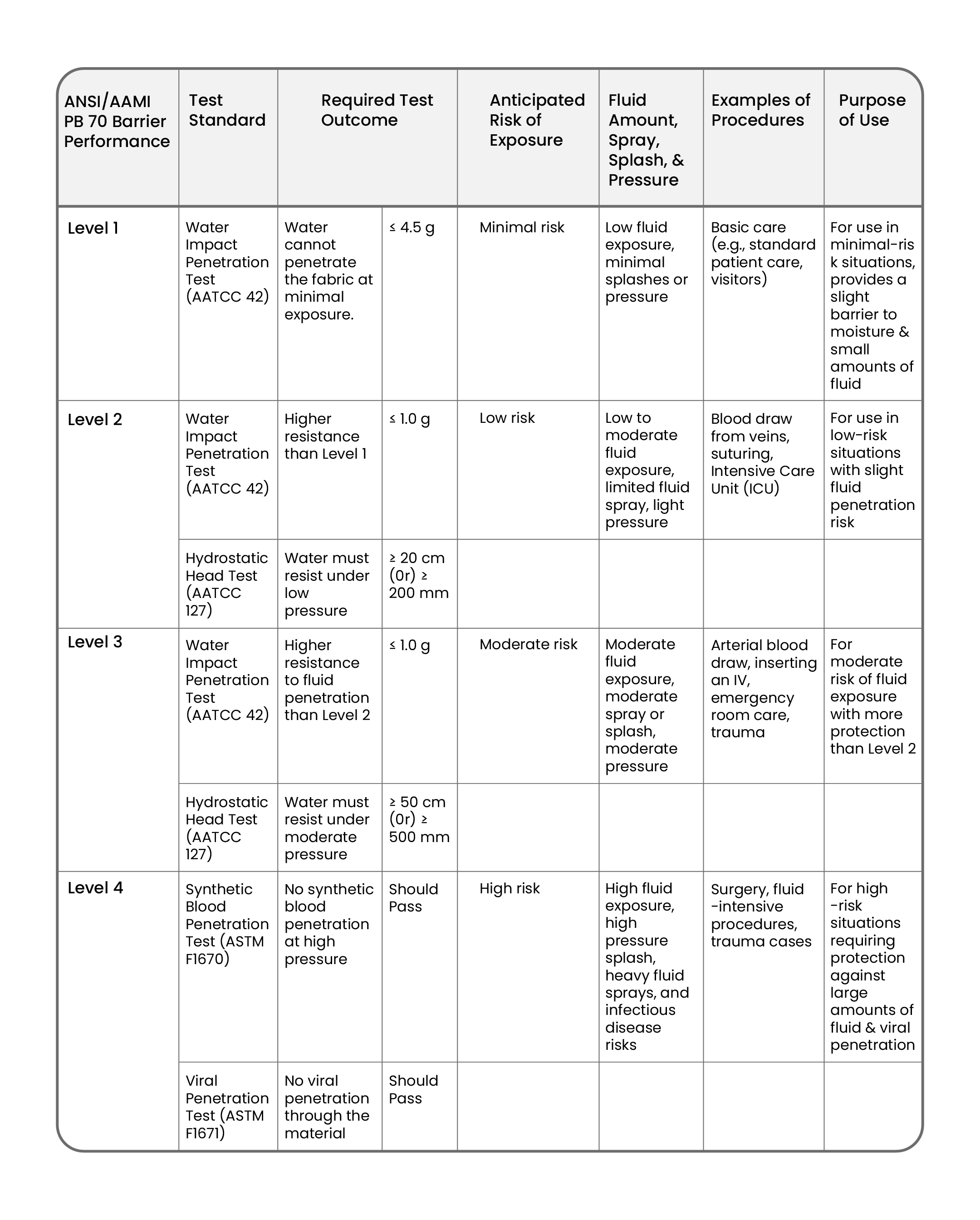
For more details on AAMI gown levels, you can review the official guidelines here.
CDC Guidelines for Personal Protective Equipment (PPE)
The Centers for Disease Control and Prevention (CDC) also offers important guidelines for the proper selection, use, and disposal of surgical gowns and other personal protective equipment (PPE) in healthcare settings. These guidelines help ensure the safety of healthcare workers when handling infectious materials.
For additional information on CDC guidelines, click here.
Amaryllis Healthcare: Pioneers in Surgical Gown Manufacturing
At Amaryllis Healthcare, we take pride in producing high-quality surgical gowns in our Class 100k cleanroom facility. Our gowns are designed to meet international standards, ensuring safety, comfort, and barrier protection.
In-House Sterilization
All our gowns undergo Ethylene Oxide (EO) sterilization as per ISO 11135 standards, ensuring they are free from harmful microorganisms.
Medical-Grade Packaging
We use packaging materials that comply with ISO 11607-1 and EN 868-5, guaranteeing the sterility and integrity of our products until they reach the user.
Non-Woven Fabrics
Our gowns are made from premium non-woven fabrics that meet AAMI PB70:2003 and EN 13795 standards, providing superior protection and breathability.
Aseptic Folding
Each surgical gown is folded using 100% aseptic techniques to minimize the risk of contamination during gowning.
Conclusion
Selecting the right surgical gown is essential for ensuring the safety of both healthcare providers and patients during procedures. Disposable surgical gowns, with their high level of infection prevention, offer a better choice by reducing contamination risks. Careful consideration of factors like material composition, AAMI protection levels, and critical zones will ensure that the gown chosen meets the specific demands of the surgical environment.
At Amaryllis Healthcare, we are committed to delivering premium surgical gowns that meet the highest international safety standards. With years of expertise in surgical manufacturing, we ensure that every gown undergoes a rigorous sterilization process and is packaged with the utmost care, providing optimal protection for healthcare teams. As an ISO 13485 certified company, we adhere to strict quality management systems, while our European CE and CDSCO Class B certifications further validate the superior quality and safety of our products. We take pride in offering gowns that healthcare professionals can trust for their protection and performance.
By adhering to global guidelines and maintaining the highest standards of manufacturing, we are happy to be at the forefront of surgical gown innovation and production.
If you’d like to know more about our products, email us at [email protected].
Sources:
Featured Posts
Things you need to know about surgical gowns

